- Question: What are the main characteristics of electromagnetic radiation?
Answer: Electromagnetic radiation is characterized by its wavelength, frequency, amplitude, and speed. It travels at the speed of light (c = 3.00 x 10^8 m/s) in a vacuum. The wavelength (λ) and frequency (ν) are related by the equation c = λν.
- Question: Describe the photoelectric effect and its significance.
Answer: The photoelectric effect is the emission of electrons from a metal surface when it is exposed to light of a certain frequency. It provided evidence for the particle nature of light and led to the development of quantum mechanics. Einstein explained it by proposing that light consists of quanta (photons) with energy E = hν, where h is Planck’s constant.
- Question: What is the significance of the hydrogen atom’s emission spectrum?
Answer: The hydrogen atom’s emission spectrum consists of discrete lines, each corresponding to a specific energy transition. It provides evidence for quantized energy levels in atoms and supports the Bohr model of the atom.
- Question: What are the main postulates of the Bohr model of the hydrogen atom?
Answer: Bohr’s model postulates that electrons orbit the nucleus in specific, quantized orbits without radiating energy, and energy is only emitted or absorbed when an electron transitions between these orbits. The energy of these orbits is given by

- Question: Derive the expression for the radius of the nth orbit in the Bohr model.
Answer: The radius rn of the nth orbit is given by
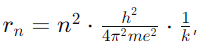
where h is Planck’s constant, m is the electron mass, e is the electron charge, and k is Coulomb’s constant.
- Question: What are the main limitations of Bohr’s model of the atom?
Answer: Bohr’s model could not explain the spectra of atoms with more than one electron, the Zeeman effect, or the Stark effect. It also did not account for the wave nature of electrons or the principles of quantum mechanics.
- Question: State de Broglie’s hypothesis.
Answer: De Broglie proposed that particles, such as electrons, exhibit wave-like properties and that the wavelength λ associated with a particle is given by

where h is Planck’s constant, m is the mass of the particle, and v is its velocity.
- Question: What does the Heisenberg uncertainty principle state?
Answer: The Heisenberg uncertainty principle states that it is impossible to simultaneously determine the exact position and momentum of a particle. Mathematically, it is expressed as

is the uncertainty in momentum.
- Question: What are the key features of the quantum mechanical model of the atom?
Answer: The quantum mechanical model describes electrons as wave functions rather than particles in fixed orbits. It incorporates the principles of wave-particle duality and the uncertainty principle, and it uses quantum numbers to describe electron distributions and energies.
- Question: What are atomic orbitals?
Answer: Atomic orbitals are regions in space where the probability of finding an electron is highest. They are described by wave functions (ψ) and characterized by quantum numbers.
- Question: How does the probability density function (ψ²) vary for 1s and 2s orbitals?
Answer: For a 1s orbital, ψ² decreases exponentially with distance from the nucleus, showing a maximum at the nucleus. For a 2s orbital, ψ² has a radial node where the probability is zero, and it increases again before finally decreasing.
- Question: What are the four quantum numbers and their significance?
Answer: The four quantum numbers are:
- Principal quantum number (n): Indicates the energy level and size of the orbital.
- Angular momentum quantum number (l): Indicates the shape of the orbital.
- Magnetic quantum number (m_l): Indicates the orientation of the orbital.
- Spin quantum number (m_s): Indicates the spin of the electron.
- Question: Describe the shapes of s, p, and d orbitals.
Answer:
- s orbitals are spherical.
- p orbitals are dumbbell-shaped with three orientations (px, py, pz).
- d orbitals have more complex shapes, including cloverleaf patterns and a donut-shaped region.
- Question: What is electron spin, and what is the spin quantum number?
Answer: Electron spin is an intrinsic form of angular momentum carried by electrons. The spin quantum number (m_s) can have values of +1/2 or -1/2, indicating the two possible spin states of an electron.
- Question: State the Aufbau principle.
Answer: The Aufbau principle states that electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels.
- Question: What is Pauli’s exclusion principle?
Answer: Pauli’s exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
- Question: Explain Hund’s rule.
Answer: Hund’s rule states that electrons will fill degenerate orbitals (orbitals with the same energy) singly and with parallel spins before pairing up.
- Question: Write the electronic configuration of oxygen (atomic number 8).
Answer: The electronic configuration of oxygen is 1s² 2s² 2p⁴.
- Question: Why are half-filled and completely filled orbitals more stable?
Answer: Half-filled and completely filled orbitals have lower energy and increased stability due to symmetrical distribution of electrons and exchange energy, which arises from electron repulsion being minimized.
- Question: Describe the significance of the quantum mechanical model in modern chemistry.
Answer: The quantum mechanical model provides a comprehensive framework for understanding the behavior of electrons in atoms, predicting atomic properties, explaining chemical bonding, and the electronic structure of molecules, which is crucial for the development of new materials and understanding chemical reactions.