Understand the concepts of Atoms and Nuclei including atomic models, radioactivity, nuclear reactions, and mass-energy equivalence. Essential for JEE, NEET & CBSE Physics preparation.
Table of Contents
Atoms and nuclei question bank
1. What was the purpose of the alpha-particle scattering experiment?
Answer: The purpose of the alpha-particle scattering experiment, conducted by Rutherford, was to study the structure of the atom by observing how alpha particles are deflected when they pass through a thin gold foil.
2. Describe Rutherford’s model of the atom.
Answer: Rutherford’s model of the atom proposes that atoms have a small, dense, positively charged nucleus at the center, with electrons orbiting around the nucleus in empty space.
3. What were the key observations from Rutherford’s alpha-particle scattering experiment?
Answer: The key observations were that most alpha particles passed through the foil without deflection, some were deflected at small angles, and a few were deflected back at large angles, indicating the presence of a dense, positively charged nucleus.
4. Explain the Bohr model of the atom.
Answer: The Bohr model suggests that electrons orbit the nucleus in fixed paths called energy levels without radiating energy. Electrons can move between these levels by absorbing or emitting energy in discrete amounts called quanta.
5. What are energy levels in the context of the Bohr model?
Answer: Energy levels are the fixed orbits around the nucleus where electrons reside. Each level corresponds to a specific energy, and electrons can transition between levels by absorbing or emitting photons of specific energies.
6. What is the hydrogen spectrum?
Answer: The hydrogen spectrum consists of the series of spectral lines emitted by hydrogen atoms when their electrons transition between energy levels. It includes the Lyman, Balmer, Paschen, Brackett, and Pfund series.
7. What is the composition of the nucleus?
Answer: The nucleus is composed of protons, which are positively charged particles, and neutrons, which are neutral particles. Together, these are called nucleons.
8. How is the size of the nucleus determined?
Answer: The size of the nucleus is determined by measuring the scattering of high-energy particles off nuclei or by analyzing the binding energy and mass defect. Typically, the radius of a nucleus is given by the formula
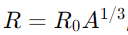
where R0 is a constant (about 1.2-1.3 fm) and A is the mass number.
9. Define atomic mass.
Answer: Atomic mass is the mass of an atom, usually expressed in atomic mass units (u), where 1 atomic mass unit is defined as one-twelfth of the mass of a carbon-12 atom.
10. Explain the mass-energy relation.
Answer: The mass-energy relation, given by Einstein’s equation
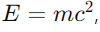
states that mass can be converted into energy and vice versa. Here, E is the energy, m is the mass, and c is the speed of light.
11. What is mass defect?
Answer: Mass defect is the difference between the sum of the masses of individual nucleons and the actual mass of the nucleus. It represents the mass converted into binding energy to hold the nucleus together.
12. How is binding energy per nucleon calculated?
Answer: Binding energy per nucleon is calculated by dividing the total binding energy of the nucleus by the number of nucleons (protons and neutrons) in the nucleus.
13. Describe the variation of binding energy per nucleon with mass number.
Answer: Binding energy per nucleon generally increases with mass number, reaches a maximum around iron (A ≈ 56), and then gradually decreases for heavier nuclei. This variation explains the stability of different nuclei.
14. What is nuclear fission?
Answer: Nuclear fission is the process in which a heavy nucleus splits into two or more lighter nuclei, accompanied by the release of a significant amount of energy and neutrons.
15. Give an example of a nuclear fission reaction.
Answer: An example of a nuclear fission reaction is the splitting of uranium-235 when it absorbs a neutron:

16. What is nuclear fusion?
Answer: Nuclear fusion is the process in which two light nuclei combine to form a heavier nucleus, releasing a large amount of energy. This is the process that powers the sun and other stars.
17. Give an example of a nuclear fusion reaction.
Answer: An example of a nuclear fusion reaction is the fusion of deuterium (heavy hydrogen) and tritium (superheavy hydrogen):

18. Why is binding energy important in nuclear reactions?
Answer: Binding energy is important because it determines the stability of a nucleus and the energy released or absorbed during nuclear reactions. Higher binding energy per nucleon means a more stable nucleus.
19. How does the concept of mass defect support the mass-energy relation?
Answer: Mass defect supports the mass-energy relation by demonstrating that the missing mass of a nucleus (mass defect) has been converted into binding energy, consistent with
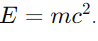
20. Why is the study of nuclear fission and fusion important for energy production?
Answer: The study of nuclear fission and fusion is important for energy production because these processes release enormous amounts of energy, which can be harnessed for generating electricity, providing a potential solution to the world’s energy needs.
Atoms and Nuclei is a crucial part of Modern Physics that connects classical atomic theories with nuclear phenomena. It carries significant weightage in competitive exams like JEE Main, NEET, and CBSE Class 12 Physics.
Introduction to Atoms and Nuclei
Atoms are the basic building blocks of matter, consisting of a nucleus surrounded by electrons. The nucleus, in turn, contains protons and neutrons, and is the source of nuclear energy and radioactivity.
This article covers the structure of the atom, Bohr’s model, and the phenomena of radioactivity, along with the laws of radioactive decay and Einstein’s mass-energy equivalence.
Key Topics in Atoms and Nuclei
📌 Atoms
- Rutherford’s Scattering Experiment
- Rutherford’s Nuclear Model of the Atom
- Bohr’s Postulates and Hydrogen Spectrum
- Energy Levels and Line Spectra
- Limitations of Bohr’s Model
📌 Nuclei
- Composition of Nucleus: Protons, Neutrons (Nucleons)
- Nuclear Size and Mass
- Isotopes, Isobars, and Isotones
- Nuclear Binding Energy and Mass Defect
- Radioactivity: Alpha, Beta, and Gamma Decay
- Law of Radioactive Decay & Decay Constant
- Half-life and Mean Life
- Nuclear Fission and Fusion
- Einstein’s Equation: E = mc²
Importance of Atoms and Nuclei in JEE & NEET
- Frequently appears in Modern Physics section of JEE & NEET
- Conceptual + numerical questions in radioactive decay, binding energy, and mass defect
- Easy to score with conceptual clarity and formula-based practice
- One of the high-yield chapters for NEET Physics
Preparation Tips
- Memorize standard values like nuclear radius and Avogadro’s number
- Solve previous year questions to identify patterns
- Focus on numerical questions involving radioactive decay, binding energy, and Bohr’s formula
- Use visual aids like atomic structure diagrams and decay chains
Important Formulas
| Concept | Formula |
|---|---|
| Radius of nucleus | R = R₀ × A^(1/3) |
| Binding Energy (BE) | BE = [Z×mₚ + (A–Z)×mₙ – mₙucleus]c² |
| Radioactive Decay Law | N = N₀ × e^(–λt) |
| Half-life | T½ = 0.693 / λ |
| Energy-Mass Relation | E = mc² |
Summary Table
| Term | Description |
|---|---|
| Nucleus | Central dense region of an atom containing protons and neutrons |
| Radioactivity | Spontaneous emission of particles or rays by unstable nuclei |
| Binding Energy | Energy required to disassemble a nucleus into protons and neutrons |
| Fission | Splitting of a heavy nucleus into smaller nuclei |
| Fusion | Combining of lighter nuclei to form a heavier nucleus |
Recommended Resources
- NCERT Class 12 Physics – Chapter: Atoms & Nuclei
- HC Verma Part 2 – Modern Physics
- Practice sets from coaching institutes like thinkIIT-thinkNEET, Aakash, Allen
- Online test series and MCQs from MyExams.Ai
Powered by CBSE Study Hub. Your Free Study Partner.