Kossel-Lewis Approach to Chemical Bond Formation
- Question: What is the Kossel-Lewis approach to chemical bonding?
Answer: The Kossel-Lewis approach to chemical bonding involves the concept of atoms achieving a stable electronic configuration, typically an octet, by either transferring or sharing electrons to form chemical bonds.
- Question: How do ionic and covalent bonds differ according to the Kossel-Lewis approach?
Answer: Ionic bonds are formed by the complete transfer of electrons from one atom to another, resulting in the formation of positively and negatively charged ions. Covalent bonds are formed by the sharing of electrons between atoms to achieve a stable electronic configuration.
Ionic Bonding
- Question: What factors affect the formation of ionic bonds?
Answer: Factors affecting the formation of ionic bonds include the ionization energy of the metal, electron affinity of the non-metal, lattice enthalpy of the compound, and the relative sizes and charges of the ions.
- Question: How is lattice enthalpy calculated?
Answer: Lattice enthalpy is calculated using the Born-Haber cycle, which involves the combination of ionization energy, electron affinity, sublimation energy, and bond dissociation energy, along with the energy change associated with forming the ionic crystal from gaseous ions.
Covalent Bonding
- Question: Define electronegativity and its role in covalent bonding.
Answer: Electronegativity is the ability of an atom to attract shared electrons in a covalent bond. It determines the polarity of the bond, with greater differences in electronegativity leading to more polar covalent bonds.
- Question: What is Fajan’s rule?
Answer: Fajan’s rule states that the degree of covalent character in an ionic bond increases with the increase in polarization of the anion by the cation. Factors such as the size and charge of the cation and anion influence this polarization.
- Question: What is a dipole moment?
Answer: A dipole moment is a measure of the separation of positive and negative charges in a molecule, indicating the polarity of a bond. It is calculated as the product of the charge magnitude and the distance between the charges.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- Question: Describe the VSEPR theory.
Answer: VSEPR theory states that the shape of a molecule is determined by the repulsion between electron pairs in the valence shell of the central atom. Electron pairs arrange themselves to minimize repulsion, leading to specific molecular geometries.
- Question: What is the shape of a molecule with a steric number of 4 and no lone pairs?
Answer: A molecule with a steric number of 4 and no lone pairs has a tetrahedral shape.
Quantum Mechanical Approach to Covalent Bonding
- Question: What is the valence bond theory?
Answer: Valence bond theory describes the formation of covalent bonds as the overlap of atomic orbitals, with shared electron pairs occupying the overlapping region. This theory emphasizes the localized nature of electrons in bonds.
- Question: Explain the concept of hybridization.
Answer: Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It explains the geometry of covalent bonds in molecules. Common types include sp, sp², and sp³ hybridization.
- Question: What is resonance in chemistry?
Answer: Resonance is the phenomenon where a molecule can be represented by two or more equivalent Lewis structures, called resonance structures. The true structure is a hybrid of these structures, resulting in delocalized electrons.
Molecular Orbital Theory
- Question: What are the main features of molecular orbital theory?
Answer: Molecular orbital theory describes the combination of atomic orbitals to form molecular orbitals, which are spread over the entire molecule. Electrons in these orbitals can be bonding, antibonding, or non-bonding.
- Question: What is the difference between bonding and antibonding molecular orbitals?
Answer: Bonding molecular orbitals are lower in energy and result from constructive interference of atomic orbitals, leading to electron density between nuclei. Antibonding molecular orbitals are higher in energy due to destructive interference, reducing electron density between nuclei.
- Question: How is bond order calculated in molecular orbital theory?
Answer: Bond order is calculated as (number of electrons in bonding orbitals – number of electrons in antibonding orbitals) / 2. A higher bond order indicates a stronger bond.
- Question: What is the molecular orbital configuration of oxygen (O₂)?
Answer: The molecular orbital configuration of O₂ is
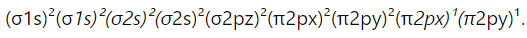
Other Types of Bonding
- Question: What is metallic bonding?
Answer: Metallic bonding is the electrostatic attraction between a lattice of positively charged metal ions and a sea of delocalized valence electrons. This bonding gives metals their characteristic properties such as conductivity and malleability.
- Question: Define hydrogen bonding and its significance.
Answer: Hydrogen bonding is a strong type of dipole-dipole interaction occurring between a hydrogen atom bonded to a highly electronegative atom (like O, N, or F) and a lone pair of electrons on another electronegative atom. It significantly affects the physical properties of compounds, such as the high boiling point of water.
Advanced Concepts
- Question: What is the significance of bond length and bond energy in molecular structure?
Answer: Bond length is the average distance between nuclei of two bonded atoms. Bond energy is the energy required to break a bond. Shorter bond lengths generally correspond to stronger bonds with higher bond energies, affecting the stability and reactivity of molecules.
- Question: Explain the concept of Linear Combination of Atomic Orbitals (LCAO) in molecular orbital theory.
Answer: LCAO is a method for constructing molecular orbitals by combining atomic orbitals of the bonded atoms. The resulting molecular orbitals can be bonding, antibonding, or non-bonding, and they describe the electron distribution in a molecule.
These questions and answers cover the essential concepts and principles of chemical bonding and molecular structure, providing a comprehensive overview suitable for study and review.