1. What is the rate of a chemical reaction?
Answer: The rate of a chemical reaction is the change in concentration of reactants or products per unit time.
2. How does concentration affect the rate of reactions?
Answer: An increase in the concentration of reactants generally increases the rate of reaction as more reactant molecules are available to collide and react.
3. How does pressure affect the rate of reactions involving gases?
Answer: Increasing the pressure of gases increases the rate of reaction by increasing the number of collisions between reactant molecules.
4. How does temperature affect the rate of reactions?
Answer: Raising the temperature increases the rate of reaction by providing more energy to the reactant molecules, thus increasing the number of effective collisions.
5. What role does a catalyst play in a chemical reaction?
Answer: A catalyst speeds up a chemical reaction by lowering the activation energy, allowing more reactant molecules to collide with sufficient energy to react.
6. Differentiate between elementary and complex reactions.
Answer: Elementary reactions occur in a single step and involve a direct interaction between reactants, while complex reactions occur in multiple steps involving intermediate species.
7. Define the rate law of a reaction.
Answer: The rate law is an expression that relates the rate of a reaction to the concentration of reactants, typically in the form
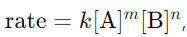
where k is the rate constant and m, n are the reaction orders.
8. What is the molecularity of a reaction?
Answer: Molecularity is the number of reactant molecules involved in an elementary reaction step. It can be unimolecular, bimolecular, or termolecular.
9. What is the rate constant and its units?
Answer: The rate constant (k) is a proportionality constant in the rate law. Its units vary depending on the reaction order:
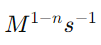
for an nth-order reaction.
10. Explain the Arrhenius equation.
Answer: The Arrhenius equation,
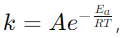
relates the rate constant k to the temperature T, activation energy Ea, and a pre-exponential factor A.
11. What is activation energy?
Answer: Activation energy (Ea) is the minimum energy that reacting molecules must possess for a reaction to occur.
12. How can activation energy be calculated?
Answer: Activation energy can be calculated from the Arrhenius equation using the slope of a plot of ln(k) versus 1/T.
13. What is collision theory?
Answer: Collision theory states that for a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation.
14. Describe zero-order reactions and their characteristics.
Answer: In zero-order reactions, the rate is independent of the concentration of reactants. The rate law is rate = k, and the half-life decreases with decreasing concentration.
15. Describe first-order reactions and their characteristics.
Answer: In first-order reactions, the rate is directly proportional to the concentration of one reactant. The rate law is rate = k[A], and the half-life is constant and independent of the initial concentration.
16. What is the half-life of a reaction?
Answer: The half-life of a reaction is the time required for the concentration of a reactant to decrease to half its initial value.
17. How does temperature affect the rate constant according to the Arrhenius theory? –
Answer: According to the Arrhenius theory, the rate constant increases exponentially with an increase in temperature.
18. What is the effect of a catalyst on activation energy?
Answer: A catalyst lowers the activation energy of a reaction, allowing more reactant molecules to have enough energy to react.
19. What is a bimolecular reaction in the context of collision theory?
Answer: A bimolecular reaction involves collisions between two reactant molecules, which must collide with sufficient energy and proper orientation to react.
20. Explain the significance of the pre-exponential factor (A) in the Arrhenius equation. –
Answer: The pre-exponential factor (A) represents the frequency of collisions with the correct orientation for a reaction to occur.