1. What is meant by the dual nature of radiation?
Answer: The dual nature of radiation refers to the concept that electromagnetic radiation, such as light, exhibits both wave-like and particle-like properties. This means light can behave as a wave in some experiments and as a particle (photon) in others.
2. What is the photoelectric effect?
Answer: The photoelectric effect is the phenomenon where electrons are ejected from the surface of a material when it is exposed to light of a certain frequency. This effect demonstrates the particle nature of light.
3. Who first observed the photoelectric effect?
Answer: The photoelectric effect was first observed by Heinrich Hertz in 1887.
4. What were Lenard’s observations regarding the photoelectric effect?
Answer: Philipp Lenard observed that the energy of ejected electrons depended on the frequency of the incident light, not its intensity, and that there was a threshold frequency below which no electrons were ejected.
5. State Einstein’s photoelectric equation.
Answer: Einstein’s photoelectric equation is given by
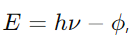
where E is the kinetic energy of the ejected electron, h is Planck’s constant, ν is the frequency of the incident light, and ϕ is the work function of the material.
6. What is the significance of Einstein’s photoelectric equation?
Answer: Einstein’s photoelectric equation provided evidence for the particle nature of light, introducing the concept of photons and explaining how energy is quantized in discrete packets.
7. Define the work function of a material.
Answer: The work function is the minimum energy required to eject an electron from the surface of a material. It is a characteristic property of the material.
8. What is a photon?
Answer: A photon is a quantum of electromagnetic radiation. It is the fundamental particle of light, carrying energy proportional to its frequency, given by
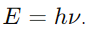
9. What is meant by the wave nature of particles?
Answer: The wave nature of particles refers to the concept that particles such as electrons exhibit wave-like properties, such as interference and diffraction, similar to light waves.
10. Who proposed the concept of matter waves?
Answer: The concept of matter waves was proposed by Louis de Broglie in 1924.
11. State the de Broglie relation.
Answer: The de Broglie relation is given by
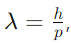
where λ is the wavelength associated with a particle, h is Planck’s constant, and p is the momentum of the particle.
12. How did de Broglie contribute to quantum mechanics?
Answer: De Broglie introduced the idea that particles, such as electrons, have wave-like properties, leading to the development of wave mechanics and significantly contributing to the foundation of quantum mechanics.
13. Explain the significance of the de Broglie wavelength.
Answer: The de Broglie wavelength explains the wave-like behavior of particles, allowing for the prediction of phenomena such as electron diffraction and interference, which are fundamental to quantum mechanics.
14. What experiment confirmed the wave nature of electrons?
Answer: The Davisson-Germer experiment in 1927 confirmed the wave nature of electrons by demonstrating electron diffraction from a crystal of nickel.
15. Describe the Davisson-Germer experiment.
Answer: In the Davisson-Germer experiment, a beam of electrons was directed at a nickel crystal. The scattered electrons formed a diffraction pattern, similar to the pattern produced by X-rays, confirming the wave nature of electrons.
16. What is Planck’s constant, and what is its value?
Answer: Planck’s constant (h) is a fundamental constant in quantum mechanics that relates the energy of a photon to its frequency. Its value is approximately
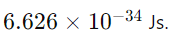
17. How does the photoelectric effect support the particle nature of light?
Answer: The photoelectric effect supports the particle nature of light by demonstrating that light can transfer energy in discrete packets (photons) to electrons, causing their ejection from a material.
18. What is the threshold frequency in the context of the photoelectric effect?
Answer: The threshold frequency is the minimum frequency of incident light required to eject electrons from a material. Below this frequency, no electrons are ejected regardless of the light’s intensity.
19. How did Einstein’s explanation of the photoelectric effect influence the development of quantum theory?
Answer: Einstein’s explanation of the photoelectric effect provided strong evidence for the quantization of energy and the existence of photons, which were pivotal in the development of quantum theory and the understanding of light and matter interactions.
20. Summarize the dual nature of matter and radiation.
Answer: The dual nature of matter and radiation refers to the concept that both light and particles exhibit both wave-like and particle-like properties. This duality is fundamental to quantum mechanics and is evidenced by phenomena such as the photoelectric effect (particle nature) and electron diffraction (wave nature).