1. What is the equation of state of a perfect gas?
Answer: The equation of state of a perfect gas is given by the ideal gas law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the universal gas constant, and T is the absolute temperature.
2. How is work done on compressing a gas related to its pressure and volume changes?
Answer: Work done on compressing a gas is given by the equation W = -PΔV, where W is work done, P is pressure, and ΔV is the change in volume. The negative sign indicates work done on the system (compression).
3. What are the assumptions of the Kinetic Theory of Gases?
Answer: The assumptions include:
- Gas particles are in constant, random motion.
- Gas particles are point masses with negligible volume.
- Collisions between gas particles and container walls are perfectly elastic.
- There are no intermolecular forces between gas particles except during collisions.
- The average kinetic energy of gas particles is directly proportional to the temperature.
4. Define the concept of pressure in the context of gases.
Answer: Pressure in gases is the force exerted by gas molecules per unit area on the walls of the container. It arises from the collisions of gas particles with the container walls.
5. How is temperature interpreted in the Kinetic Theory of Gases?
Answer: In the Kinetic Theory of Gases, temperature is interpreted as the average kinetic energy of gas molecules. Higher temperature corresponds to higher average kinetic energy.
6. What is the root mean square (RMS) speed of gas molecules?
Answer: The root mean square speed of gas molecules is the square root of the average of the squares of the speeds of all gas molecules in a sample. It is given by the equation:
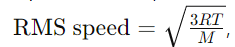
where R is the gas constant, T is the temperature in Kelvin, and M is the molar mass of the gas.
7. What are degrees of freedom in the context of kinetic theory?
Answer: Degrees of freedom refer to the number of independent ways a molecule can move in space. In kinetic theory, for a monatomic gas molecule, there are three translational degrees of freedom.
8. What is the Law of Equipartition of Energy, and how does it apply to specific heat capacities of gases?
Answer: The Law of Equipartition of Energy states that each degree of freedom of a molecule has an average energy of 1/2 kT, where k is Boltzmann’s constant and T is the temperature in Kelvin. For gases, this law explains the relationship between specific heat capacities and the degrees of freedom.
9. How is the mean free path defined in the context of gas molecules?
Answer: The mean free path is the average distance a gas molecule travels between collisions with other molecules. It depends on factors such as pressure, temperature, and molecular size.
10. What is Avogadro’s number, and how is it relevant to the Kinetic Theory of Gases?
Answer: Avogadro’s number (6.022 x 10^23) is the number of particles in one mole of a substance. In the context of the Kinetic Theory of Gases, it helps relate the macroscopic properties of gases to their microscopic behavior.
11. Explain how the equation of state of a perfect gas is derived from the Kinetic Theory of Gases.
Answer: The equation of state of a perfect gas, PV = nRT, can be derived from the Kinetic Theory of Gases by considering the average kinetic energy of gas molecules and their collisions with the container walls.
12. Discuss the significance of the ideal gas law in understanding gas behavior.
Answer: The ideal gas law provides a simple yet accurate description of gas behavior under many conditions. It allows for the prediction of gas properties such as pressure, volume, and temperature based on the amount of gas present.
13. How does increasing the temperature affect the RMS speed of gas molecules?
Answer: Increasing the temperature increases the average kinetic energy of gas molecules, leading to an increase in their RMS speed. This relationship is described by the equation for RMS speed.
14. Describe the relationship between pressure and the number of gas molecules in a container according to the Kinetic Theory of Gases.
Answer: According to the Kinetic Theory of Gases, pressure is directly proportional to the number of gas molecules present in a container. Increasing the number of molecules increases the frequency of collisions with the container walls, resulting in higher pressure.
15. How do the assumptions of the Kinetic Theory of Gases help explain the behavior of real gases?
Answer: While real gases deviate from ideal behavior under certain conditions, the assumptions of the Kinetic Theory of Gases provide a useful framework for understanding gas behavior. By considering gas particles as point masses in constant motion, the theory can explain many properties of real gases.
16. Explain why the specific heat capacity of gases varies with temperature and pressure.
Answer: The specific heat capacity of gases varies with temperature and pressure due to changes in the kinetic energy and collision frequency of gas molecules. At higher temperatures and pressures, gas molecules have greater kinetic energy and collide more frequently, leading to changes in specific heat capacity.
17. How does the Law of Equipartition of Energy explain the relationship between temperature and kinetic energy?
Answer: The Law of Equipartition of Energy states that each degree of freedom of a molecule has an average energy of 1/2 kT. As temperature increases, the average kinetic energy of gas molecules also increases, consistent with the kinetic interpretation of temperature.
18. Discuss the concept of thermal conductivity and its relationship to the mean free path of gas molecules.
Answer: Thermal conductivity is a measure of a material’s ability to conduct heat. In gases, thermal conductivity depends on factors such as temperature, pressure, and the mean free path of gas molecules. A longer mean free path results in higher thermal conductivity.
19. How does Avogadro’s number relate to the number of gas molecules in a given volume?
Answer: Avogadro’s number represents the number of particles in one mole of a substance. In the context of gases, it relates the number of gas molecules to the volume occupied by the gas at a given temperature and pressure.
20. Discuss the significance of the mean free path in understanding gas behavior and properties. Answer: The mean free path is important in understanding gas behavior and properties because it determines the frequency and nature of collisions between gas molecules. It affects phenomena such as diffusion, thermal conductivity, and viscosity in gases.