1. Define the rate of a chemical reaction and explain how it is measured.
Answer: The rate of a chemical reaction is the change in concentration of reactants or products per unit time. It is measured by monitoring the change in concentration over a period of time.
2. Discuss the factors affecting the rate of chemical reactions.
Answer: Factors affecting reaction rate include concentration, temperature, pressure (for gases), and the presence of a catalyst.
3. Explain the concept of elementary and complex reactions in chemical kinetics.
Answer: Elementary reactions occur in a single step and involve only a few molecules, while complex reactions occur in multiple steps and involve the formation of intermediate species.
4. Define the order and molecularity of reactions.
Answer: The order of a reaction is the sum of the exponents in the rate law equation, while the molecularity refers to the number of molecules or ions that participate in the elementary step of a reaction.
5. What is the rate law of a chemical reaction?
Answer: The rate law is an equation that relates the rate of a reaction to the concentrations of reactants raised to various powers, as determined experimentally.
6. Define the rate constant and discuss its units.
Answer: The rate constant (k) is the proportionality constant in the rate law equation. Its units depend on the overall order of the reaction.
7. Describe the differential and integral forms of zero and first-order reactions.
Answer:
> For zero-order reactions, the differential form is
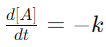
and the integral form is
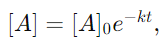
> For first-order reactions, the differential form is
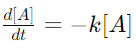
and the integral form is

8. Discuss the characteristics and half-lives of zero and first-order reactions.
Answer:
- Zero-order reactions have a constant half-life, independent of initial concentration.
- First-order reactions have a constant half-life, which is inversely proportional to the initial concentration.
9. How does temperature affect the rate of chemical reactions?
Answer: Increasing temperature generally increases the rate of chemical reactions by providing more kinetic energy to the molecules, leading to more frequent and energetic collisions.
10. Explain the Arrhenius theory and its significance in chemical kinetics.
Answer: The Arrhenius theory relates the rate constant of a reaction to temperature and activation energy. It helps explain the exponential relationship between reaction rate and temperature.
11. Define activation energy and discuss methods for its calculation.
Answer: Activation energy (Ea) is the minimum energy required for a reaction to occur. It can be calculated using the Arrhenius equation or determined experimentally from reaction rate data at different temperatures.
12. What is the collision theory of bimolecular gaseous reactions?
Answer: The collision theory proposes that chemical reactions occur when reactant molecules collide with sufficient energy and proper orientation to overcome the activation energy barrier.
13. Explain the concept of a catalyst and its role in chemical reactions.
Answer: A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with lower activation energy. It is not consumed in the reaction and does not appear in the overall stoichiometry.
14. How does concentration affect the rate of a chemical reaction?
Answer: Increasing the concentration of reactants generally increases the rate of reaction by increasing the frequency of collisions between reacting particles.
15. Discuss the effect of pressure on the rate of chemical reactions.
Answer: For gaseous reactions, increasing pressure generally increases the rate of reaction by increasing the concentration of reactant particles and hence the frequency of collisions.
16. How does the presence of a catalyst affect the rate of a chemical reaction?
Answer: A catalyst increases the rate of a chemical reaction by providing an alternative reaction pathway with lower activation energy, thereby lowering the energy barrier for the reaction to occur.
17. Explain the concept of molecularity in the context of reaction mechanisms.
Answer: Molecularity refers to the number of molecules or ions that participate in the elementary step of a reaction mechanism. It helps determine the overall rate law for a complex reaction.
18. Discuss the significance of half-life in determining reaction kinetics.
Answer: The half-life of a reaction is the time required for the concentration of a reactant to decrease to half its initial value. It provides information about the rate of decay of reactants and the stability of products.
19. What are the characteristics of complex reactions, and how are they different from elementary reactions?
Answer: Complex reactions occur in multiple steps and involve the formation of intermediate species. They often have different rate-determining steps and overall reaction orders compared to elementary reactions.
20. How does the rate constant of a reaction relate to its reaction mechanism?
Answer: The rate constant of a reaction is determined by the rate-determining step of the reaction mechanism, which is the slowest step. The overall rate law and rate constant depend on the molecularity and rate constants of individual elementary steps.