1. Define system and surroundings in thermodynamics.
Answer: The system is the part of the universe under consideration, while the surroundings are everything outside the system with which it can exchange energy or matter.
2. Differentiate between extensive and intensive properties.
Answer: Extensive properties depend on the size or amount of the system, while intensive properties are independent of the size or amount of the system.
3. What are state functions in thermodynamics?
Answer: State functions are properties that depend only on the current state of the system, such as temperature, pressure, and internal energy.
4. Define entropy.
Answer: Entropy is a measure of the disorder or randomness of a system, representing the number of possible microstates of a system at a given energy.
5. Describe the types of thermodynamic processes.
Answer: Thermodynamic processes include isothermal (constant temperature), adiabatic (no heat exchange), isobaric (constant pressure), and isochoric (constant volume) processes.
6. Explain the first law of thermodynamics.
Answer: The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. It is also known as the law of conservation of energy.
7. Define work in thermodynamics.
Answer: Work is the transfer of energy from one system to another due to a mechanical force acting through a distance.
8. What is internal energy in thermodynamics?
Answer: Internal energy is the sum of the kinetic and potential energies of the particles within a system.
9. Explain the concept of enthalpy.
Answer: Enthalpy is a state function representing the total heat content of a system at constant pressure. It is given by the sum of the internal energy and the product of pressure and volume.
10. Define heat capacity and molar heat capacity.
Answer: Heat capacity is the amount of heat required to raise the temperature of a substance by one degree Celsius or Kelvin. Molar heat capacity is the heat capacity per mole of substance.
11. Describe Hess’s law of constant heat summation.
Answer: Hess’s law states that the enthalpy change of a reaction is independent of the pathway taken, as long as the initial and final conditions are the same.
12. List and explain the different types of enthalpies.
Answer: Enthalpies include bond dissociation, combustion, formation, atomization, sublimation, phase transition, hydration, ionization, and solution enthalpies, each representing the energy change associated with a specific process.
13. What is the second law of thermodynamics?
Answer: The second law of thermodynamics states that the total entropy of a closed system and its surroundings always increases for spontaneous processes.
14. Explain spontaneity of processes in terms of thermodynamics.
Answer: Spontaneous processes are those that occur without external intervention and tend to increase the total entropy of the universe.
15. Define ΔS of the universe and ΔG of the system as criteria for spontaneity.
Answer: ΔS of the universe represents the change in total entropy of the system and surroundings, while ΔG of the system represents the change in Gibbs free energy. Spontaneous processes have ΔS > 0 for the universe and ΔG<0 for the system.
16. What is ΔG∘ (Standard Gibbs energy change)?
Answer: ΔG∘ is the Gibbs free energy change under standard conditions, typically at 1 atm pressure and 25°C temperature.
17. Explain the relationship between ΔG∘ and the equilibrium constant.
Answer: The relationship is given by
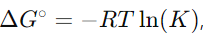
where R is the gas constant, T is the temperature in Kelvin, and K is the equilibrium constant.
18. How is the spontaneity of a reaction determined using ΔG∘?
Answer: A reaction is spontaneous if ΔG∘ is negative, indicating that the products have lower free energy than the reactants.
19. What is the significance of chemical thermodynamics in understanding reactions and processes?
Answer: Chemical thermodynamics provides fundamental principles for understanding the energetics and spontaneity of chemical reactions, guiding the design of industrial processes and the optimization of reaction conditions.
20. How does chemical thermodynamics contribute to the study of equilibrium and reaction kinetics?
Answer: Chemical thermodynamics helps determine the equilibrium position and predict the direction of reactions, while also providing insights into the rates of reactions through the relationship between free energy and activation energy.