1. Explain the electronic concepts of oxidation and reduction in redox reactions.
Answer: Oxidation involves the loss of electrons, while reduction involves the gain of electrons. In a redox reaction, one substance is oxidized (loses electrons) while another is reduced (gains electrons).
2. Define oxidation number and discuss its significance in redox reactions.
Answer: Oxidation number is a measure of the apparent charge of an atom in a compound or ion. It is used to track electron transfer in redox reactions and to assign oxidation states to atoms.
3. What are the rules for assigning oxidation numbers in chemical compounds?
Answer: Rules for assigning oxidation numbers include:
- The oxidation number of an element in its elemental form is zero.
- The oxidation number of a monatomic ion is equal to its charge.
- In compounds, the sum of oxidation numbers must equal the overall charge of the compound.
- In covalent compounds, hydrogen is assigned an oxidation number of +1, and oxygen is assigned an oxidation number of -2.
4. Discuss the balancing of redox reactions using the oxidation number method.
Answer: Balancing redox reactions involves ensuring that the number of electrons lost in oxidation is equal to the number gained in reduction. This can be achieved by assigning oxidation numbers, balancing atoms and charges, and adding appropriate coefficients to balance the reaction.
5. Explain electrolytic and metallic conduction in terms of electrochemistry.
Answer: Electrolytic conduction occurs in electrolyte solutions where ions carry charge, while metallic conduction occurs in metals where delocalized electrons move freely.
6. Define conductance in electrolytic solutions and discuss its measurement.
Answer: Conductance is a measure of the ability of a solution to conduct electricity. It is measured using a conductance cell and is inversely proportional to resistance.
7. Describe molar conductivities and their variation with concentration.
Answer: Molar conductivity (Λm) is the conductivity of a solution containing 1 mole of solute dissolved in 1 liter of solution. It decreases with increasing concentration due to ion-ion interactions.
8. Explain Kohlrausch’s law and its applications in determining molar conductivity.
Answer: Kohlrausch’s law states that the molar conductivity of an electrolyte at infinite dilution is the sum of the molar conductivities of its ions. It is used to calculate the molar conductivity of strong electrolytes at any concentration.
9. Differentiate between electrolytic and Galvanic cells.
Answer: Electrolytic cells use electrical energy to drive non-spontaneous reactions, while Galvanic cells spontaneously convert chemical energy into electrical energy.
10. Discuss the different types of electrodes used in electrochemical cells.
Answer: Electrodes can be classified as inert (such as platinum) or reactive (such as metal electrodes). They serve as sites for oxidation and reduction reactions to occur.
11. Define electrode potentials, including standard electrode potential.
Answer: Electrode potential is the potential difference between an electrode and its solution. Standard electrode potential (E∘) is the potential of an electrode measured against a standard hydrogen electrode under standard conditions.
12. Explain half-cell and cell reactions in electrochemistry.
Answer: A half-cell consists of an electrode immersed in a solution of its ions. A cell reaction occurs when two half-cells are connected, allowing electron transfer to occur between them.
13. Discuss the electromotive force (emf) of a Galvanic cell and its measurement.
Answer: The emf of a Galvanic cell is the maximum potential difference between its two electrodes when no current flows. It is measured using a voltmeter.
14. What is the Nernst equation and how is it applied in electrochemistry?
Answer: The Nernst equation relates the electrode potential of a half-cell to the concentrations of its reactants and products. It is used to calculate the cell potential under non-standard conditions.
15. Describe the relationship between cell potential and Gibbs’ energy change.
Answer: The relationship is given by
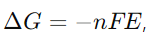
where ΔG is the Gibbs’ energy change, n is the number of moles of electrons transferred, F is the Faraday constant, and E is the cell potential.
16. Discuss the structure and function of a dry cell.
Answer: A dry cell consists of a zinc container serving as the anode, a graphite rod serving as the cathode, a paste of ammonium chloride and zinc chloride serving as the electrolyte, and a manganese dioxide paste serving as the depolarizer. It is used in portable electronic devices.
17. Explain the working principle of a lead-acid accumulator.
Answer: A lead-acid accumulator consists of lead dioxide and lead plates immersed in sulfuric acid. During discharge, lead dioxide is reduced to lead sulfate at the positive electrode, while lead is oxidized to lead sulfate at the negative electrode. During charging, the reactions are reversed.
18. What are fuel cells and how do they operate?
Answer: Fuel cells are electrochemical devices that convert chemical energy directly into electrical energy. They operate by oxidizing a fuel (such as hydrogen) at the anode and reducing an oxidant (such as oxygen) at the cathode, with an electrolyte facilitating ion transport between the electrodes.
19. Discuss the advantages and applications of fuel cells.
Answer: Fuel cells offer high efficiency, low emissions, and quiet operation. They are used in transportation (e.g., hydrogen-powered vehicles), stationary power generation, and portable electronics.
20. Explain the significance of redox reactions and electrochemistry in various fields of science and technology.
Answer: Redox reactions and electrochemistry play crucial roles in energy conversion, corrosion prevention, electroplating, industrial processes, environmental remediation, and biomedical applications. Understanding these processes is essential for advancements in numerous scientific and technological domains.