1. What are the different methods for expressing the concentration of a solution?
Answer: The methods include molality, molarity, mole fraction, percentage (by volume and mass).
2. Explain Raoult’s Law and its application to vapor pressure of solutions.
Answer: Raoult’s Law states that the vapor pressure of a solution is directly proportional to the mole fraction of solvent present in the solution.
3. Differentiate between ideal and non-ideal solutions.
Answer: Ideal solutions follow Raoult’s Law exactly, while non-ideal solutions deviate from Raoult’s Law due to intermolecular interactions between solute and solvent particles.
4. Describe the vapor pressure-composition plot for ideal and non-ideal solutions.
Answer: For ideal solutions, the plot is a straight line, while for non-ideal solutions, it curves due to deviations from Raoult’s Law.
5. What are colligative properties of dilute solutions?
Answer: Colligative properties depend on the number of solute particles in a solution, not their nature. They include the relative lowering of vapor pressure, depression of freezing point, elevation of boiling point, and osmotic pressure.
6. Explain the concept of relative lowering of vapor pressure.
Answer: The relative lowering of vapor pressure is the decrease in vapor pressure of a solvent in a solution compared to its vapor pressure as a pure solvent.
7. Describe the depression of freezing point in solutions.
Answer: The depression of freezing point is the lowering of the freezing point of a solvent when a non-volatile solute is added.
8. What causes the elevation of boiling point in solutions?
Answer: The elevation of boiling point occurs because the presence of a non-volatile solute lowers the vapor pressure of the solvent, requiring a higher temperature for the vapor pressure to equal atmospheric pressure.
9. Define osmotic pressure.
Answer: Osmotic pressure is the pressure required to stop the flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration.
10. How can the molecular mass of a solute be determined using colligative properties?
Answer: The molecular mass of a solute can be determined by measuring the colligative property (e.g., freezing point depression or boiling point elevation) and using the equation
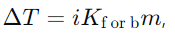
where i is the Van’t Hoff factor, Kf or b is the cryoscopic or ebullioscopic constant, and mmm is the molality of the solution.
11. What are abnormal values of molar mass in colligative properties?
Answer: Abnormal values of molar mass occur when solutes dissociate or associate in solution, leading to a Van’t Hoff factor (i) different from the expected value for non-dissociating solutes.
12. Explain the significance of the Van’t Hoff factor.
Answer: The Van’t Hoff factor indicates the degree of dissociation or association of solute particles in a solution, affecting colligative properties and helping determine the actual molar mass of solutes.
13. How does the Van’t Hoff factor differ for different types of solutes?
Answer: The Van’t Hoff factor depends on the extent of dissociation or association of solute particles. For non-dissociating solutes, i=1i = 1i=1, while for dissociating or associating solutes, iii can be greater or less than 1.
14. Explain the concept of molality and its advantages over other concentration units.
Answer: Molality is the number of moles of solute per kilogram of solvent. It is advantageous over other concentration units because it is temperature-independent and more suitable for colligative property calculations.
15. How does molarity differ from molality in expressing concentration?
Answer: Molarity is the number of moles of solute per liter of solution, while molality is the number of moles of solute per kilogram of solvent.
16. What is mole fraction and how is it calculated?
Answer: Mole fraction is the ratio of the number of moles of a component to the total number of moles in a solution. It is calculated as
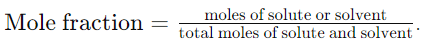
17. Describe the percentage by volume and mass in expressing solution concentration.
Answer: Percentage by volume is the volume of solute or solvent in 100 volumes of solution, while percentage by mass is the mass of solute or solvent in 100 grams of solution.
18. What are the advantages of using percentage by mass over percentage by volume in expressing solution concentration?
Answer: Percentage by mass is preferred over percentage by volume because it is less affected by temperature changes, especially for liquids with significant thermal expansion coefficients. Additionally, mass measurements are generally more accurate and reproducible than volume measurements.
19. How does the concentration of a solution affect its colligative properties?
Answer: The concentration of a solution directly affects its colligative properties, as colligative properties depend on the number of solute particles in the solution. Higher concentrations of solute lead to greater changes in colligative properties such as vapor pressure, freezing point, boiling point, and osmotic pressure.
20. Discuss the importance of Raoult’s Law in understanding solutions and predicting their behavior.
Answer: Raoult’s Law is crucial for understanding the behavior of solutions, particularly in predicting vapor pressure changes. It provides a simple model for ideal solutions, allowing us to estimate the vapor pressure of a solution based on the mole fraction of the solvent. Additionally, deviations from Raoult’s Law in non-ideal solutions offer insights into the interactions between solute and solvent molecules, leading to a deeper understanding of solution behavior.