Matter and Its Nature
- Question: What are the three states of matter?
Answer: The three states of matter are solid, liquid, and gas.
- Question: Define matter.
Answer: Matter is anything that has mass and occupies space.
- Question: What is the difference between a homogeneous mixture and a heterogeneous mixture?
Answer: A homogeneous mixture has a uniform composition throughout, whereas a heterogeneous mixture has a non-uniform composition.
Dalton’s Atomic Theory
- Question: What are the main postulates of Dalton’s atomic theory?
Answer: Dalton’s atomic theory includes the following postulates:
- Matter is composed of small indivisible particles called atoms.
- Atoms of a given element are identical in mass and properties.
- Atoms cannot be created or destroyed in chemical reactions.
- Atoms of different elements combine in simple whole-number ratios to form compounds.
- Question: Define an atom.
Answer: An atom is the smallest unit of an element that retains the properties of that element.
- Question: What is a molecule?
Answer: A molecule is a group of two or more atoms chemically bonded together.
- Question: Differentiate between an element and a compound.
Answer: An element is a pure substance consisting of only one type of atom, while a compound is a substance formed when two or more different types of atoms chemically bond together.
Laws of Chemical Combination
- Question: State the Law of Conservation of Mass.
Answer: The Law of Conservation of Mass states that mass is neither created nor destroyed in a chemical reaction.
- Question: What is the Law of Definite Proportions?
Answer: The Law of Definite Proportions states that a given chemical compound always contains its component elements in fixed ratio by mass.
- Question: Explain the Law of Multiple Proportions.
Answer: The Law of Multiple Proportions states that when two elements combine to form more than one compound, the mass of one element that combines with a fixed mass of the other element is in the ratio of small whole numbers.
Atomic and Molecular Masses
- Question: Define atomic mass.
Answer: Atomic mass is the mass of an atom, typically expressed in atomic mass units (amu).
- Question: What is molecular mass?
Answer: Molecular mass is the sum of the atomic masses of all atoms in a molecule.
Mole Concept and Molar Mass
- Question: What is a mole?
Answer: A mole is the amount of substance that contains as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12.
- Question: Define molar mass.
Answer: Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol).
- Question: How is the number of moles calculated from mass?
Answer: The number of moles is calculated by dividing the mass of the substance (in grams) by its molar mass (in g/mol).
Percentage Composition
- Question: What is percentage composition?
Answer: Percentage composition is the percent by mass of each element in a compound.
- Question: How do you calculate the percentage composition of an element in a compound?
Answer: The percentage composition of an element in a compound is calculated using the formula:
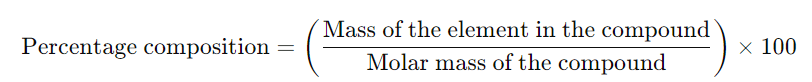
Empirical and Molecular Formulas
- Question: Define empirical formula.
Answer: The empirical formula is the simplest whole-number ratio of atoms of each element in a compound.
- Question: How is the molecular formula related to the empirical formula?
Answer: The molecular formula is a multiple of the empirical formula and represents the actual number of atoms of each element in a molecule of the compound.
Chemical Equations and Stoichiometry
- Question: What is stoichiometry?
Answer: Stoichiometry is the calculation of reactants and products in chemical reactions based on the balanced chemical equation.
- Question: How do you balance a chemical equation?
Answer: To balance a chemical equation, ensure that the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side by adjusting coefficients.
These questions cover fundamental concepts in chemistry and provide a solid foundation for understanding the basics of the subject.